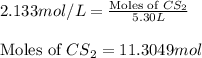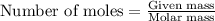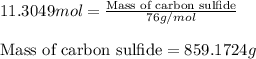Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀cs2(=9.40 at 900 k how many grams of cs2(g) can be prepared by heating 12.5 mol s2(g) with excess carbon in a 5.30 l reaction vessel held at 900 k until equilibrium is attained?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Carbon disulfide is prepared by heating sulfur and charcoal. the chemical equation is s2(g)+c(s)↽−−⇀...
Questions



Mathematics, 29.01.2021 14:00

Chemistry, 29.01.2021 14:00

Chemistry, 29.01.2021 14:00


History, 29.01.2021 14:00

Chemistry, 29.01.2021 14:00

Biology, 29.01.2021 14:00

History, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

Business, 29.01.2021 14:00



Biology, 29.01.2021 14:00

Biology, 29.01.2021 14:00


English, 29.01.2021 14:00


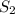 , we use the equation:
, we use the equation: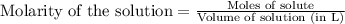 .....(1)
.....(1)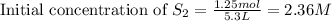
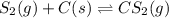
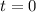 2.36
2.36 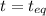 2.36 - x x
2.36 - x x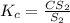

![[CS_2]=x](/tpl/images/0156/7037/d556c.png)
![[S_2]=2.36-x](/tpl/images/0156/7037/9691b.png)
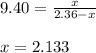
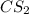 = 2.133 M
= 2.133 M