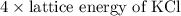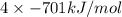Chemistry, 01.08.2019 06:10 izzyisawesome5232
Kclkcl has a lattice energy of −701 kj/mol.−701 kj/mol. consider a generic salt, abab , where a2+a2+ has the same radius as k+,k+, and b2−b2− has the same radius as xl−.xl−. estimate the lattice energy of the salt abab .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

You know the right answer?
Kclkcl has a lattice energy of −701 kj/mol.−701 kj/mol. consider a generic salt, abab , where a2+a2+...
Questions







History, 26.08.2020 01:01

Medicine, 26.08.2020 01:01





Mathematics, 26.08.2020 01:01




Computers and Technology, 26.08.2020 01:01



Physics, 26.08.2020 01:01

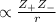
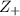 = +2, and
= +2, and  = -2
= -2