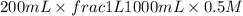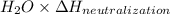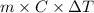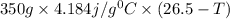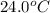Chemistry, 05.08.2019 19:10 camrenp9889
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solution is 26.5°c. what was the initial temperature of the solution before the reaction occurred? assume that the solution has a total mass of 350. g and a specific heat capacity of 4.184 j/g°c. the enthalpy of neutralization for the reaction is -62.0 kj/mol of water produced.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
You know the right answer?
When 150. ml of 0.400 m h+ are mixed with 200. ml of 0.500 m oh-, the final temperature of the solut...
Questions


Computers and Technology, 27.06.2020 05:01


Health, 27.06.2020 05:01

Health, 27.06.2020 05:01






Chemistry, 27.06.2020 05:01


Computers and Technology, 27.06.2020 05:01




Mathematics, 27.06.2020 05:01



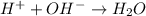
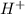 = volume × concentration of
= volume × concentration of 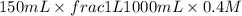
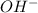 = volume × concentration of
= volume × concentration of