
Chemistry, 08.08.2019 19:10 alyxkellar06
Under certain conditions, the equilibrium constant of the reaction below is kc=1.7×10−3. if the reaction begins with a concentration of 0.0546 m for each of sbcl3 and cl2 and a concentration of 0.0 m for sbcl5, what is the equilibrium concentration (in molarity) of cl2? sbcl5(g)↽−−⇀sbcl3(g)+cl2(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Under certain conditions, the equilibrium constant of the reaction below is kc=1.7×10−3. if the reac...
Questions

Biology, 25.11.2019 01:31

Biology, 25.11.2019 01:31


Mathematics, 25.11.2019 01:31

Mathematics, 25.11.2019 01:31

History, 25.11.2019 01:31

Mathematics, 25.11.2019 01:31

History, 25.11.2019 01:31

Health, 25.11.2019 01:31


Biology, 25.11.2019 01:31


Physics, 25.11.2019 01:31


Biology, 25.11.2019 01:31

Mathematics, 25.11.2019 01:31


Mathematics, 25.11.2019 01:31

Geography, 25.11.2019 01:31

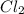 at equilibrium will be, 0.0086 M
at equilibrium will be, 0.0086 M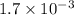
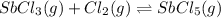
![K_c=\frac{[SbCl_5]}{[SbCl_3][Cl_2]}](/tpl/images/0173/2882/257e5.png)
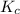 will be,
will be, 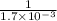
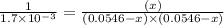
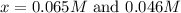
![(0.0546-x)M=[0.0546-2(0.045)]M=0.0086M](/tpl/images/0173/2882/293d4.png)


