
The decomposition of nh4hs is endothermic: nh4hs(s)⇌nh3(g)+h2s(g) part a which change to an equilibrium mixture of this reaction results in the formation of more h2s? which change to an equilibrium mixture of this reaction results in the formation of more ? a decrease in the volume of the reaction vessel (at constant temperature) an increase in the amount of nh4hs in the reaction vessel an increase in temperature all of the above

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
The decomposition of nh4hs is endothermic: nh4hs(s)⇌nh3(g)+h2s(g) part a which change to an equilib...
Questions


History, 18.03.2020 03:20









Chemistry, 18.03.2020 03:20





Social Studies, 18.03.2020 03:20

Physics, 18.03.2020 03:20


Chemistry, 18.03.2020 03:21

Mathematics, 18.03.2020 03:21

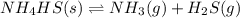
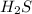 will increase.
will increase.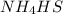 then equilibrium will shift in the direction of decrease in concentration that is, in the forward direction.
then equilibrium will shift in the direction of decrease in concentration that is, in the forward direction.

