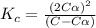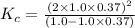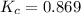Chemistry, 09.08.2019 19:20 pwolfiimp4
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium described by the equation n2o4(g) ↔ 2no2(g) if at equilibrium the n2o4 is 37% dissociated, what is the value of the equilibrium constant, kc, for the reaction under these conditions?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2


Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3
You know the right answer?
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium descr...
Questions














Mathematics, 14.11.2019 02:31




Biology, 14.11.2019 02:31


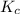 for the reaction is, 0.869
for the reaction is, 0.869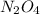 .
.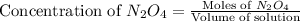
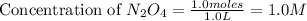
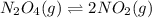
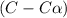
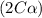
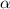 = 37 % = 0.37
= 37 % = 0.37![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0173/7935/271f5.png)