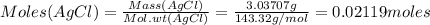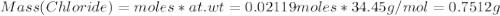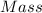Chemistry, 10.08.2019 06:10 lupitasgirl3326
A2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. the resulting precipitate, filtered and dried, weighs 3.03707 g. what was the percent by mass of chloride ion in the original compound? what is the identity of the salt?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1
You know the right answer?
A2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01