
Chemistry, 13.08.2019 02:30 tarabrett8863
The reaction a → products is first-order in a. if the concentration of a is cut in half, the half-life of the reaction will
a. double.
b. decrease by a factor of 1/2.
c. remain constant.
d. quadruple.
e. decreased by a factor of 1/4.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1
You know the right answer?
The reaction a → products is first-order in a. if the concentration of a is cut in half, the half-li...
Questions

Mathematics, 26.08.2019 21:30



English, 26.08.2019 21:30

Mathematics, 26.08.2019 21:30















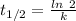
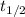 is the half life
is the half life

