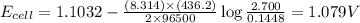Consider the reaction corresponding to a voltaic cell and its standard cell potential. z n ( s ) + c u 2 + ( a q ) ⟶ c u ( s ) + z n 2 + ( a q ) zn(s)+cux2+(aq)⟶cu(s)+znx2+(aq) e o cell = 1.1032 v ecello=1.1032 v what is the cell potential for a cell with a 2.700 m solution of z n 2 + ( a q ) znx2+(aq) and 0.1448 m solution of c u 2 + ( a q ) cux2+(aq) at 436.2 k? c

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Consider the reaction corresponding to a voltaic cell and its standard cell potential. z n ( s ) + c...
Questions

Biology, 19.09.2019 19:00




Biology, 19.09.2019 19:00



French, 19.09.2019 19:00


Biology, 19.09.2019 19:00

English, 19.09.2019 19:00

Mathematics, 19.09.2019 19:00



Mathematics, 19.09.2019 19:00


English, 19.09.2019 19:00




![E_{cell}=E^o_{cell}-\frac{RT}{nF}\log \frac{[Zn^{2+}]^2}{[Cu^{2+}]}](/tpl/images/0174/9065/fa75b.png)
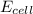 = cell potential of the cell = ?
= cell potential of the cell = ?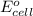 = standard electrode potential = 1.1032 V
= standard electrode potential = 1.1032 V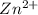 = 2.700 M
= 2.700 M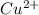 = 0.1448 M
= 0.1448 M