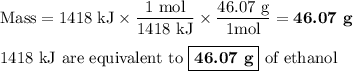Chemistry, 18.08.2019 14:10 rleiphart1
The equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into its equivalent mass. c2h5oh(l) + 3 o2(g) → 2 co2(g) + 3 h2o (l) δh = -1418 kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
The equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into...
Questions



SAT, 08.03.2021 22:30


Physics, 08.03.2021 22:30




Geography, 08.03.2021 22:30

Health, 08.03.2021 22:30



Mathematics, 08.03.2021 22:30

Mathematics, 08.03.2021 22:30





History, 08.03.2021 22:30

History, 08.03.2021 22:30