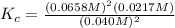Chemistry, 22.08.2019 01:10 AmityHeart
Consider the following reaction: 2nobr(g) 2no(g) + br2(g)if 0.412 moles of nobr(g), 0.678 moles of no, and 0.224 moles of br2 are at equilibrium in a 10.3 l container at 516 k, the value of the equilibrium constant, kc, is:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
Consider the following reaction: 2nobr(g) 2no(g) + br2(g)if 0.412 moles of nobr(g), 0.678 moles of n...
Questions

Arts, 23.11.2020 03:10







English, 23.11.2020 03:10

English, 23.11.2020 03:10

English, 23.11.2020 03:10

Mathematics, 23.11.2020 03:10

Mathematics, 23.11.2020 03:10





Biology, 23.11.2020 03:10



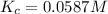
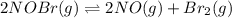
![K_c=\frac{[products]}{[reactants]}](/tpl/images/0186/4128/cdede.png)
![K_c=\frac{[NO]^2[Br_2]}{[NOBr]^2}](/tpl/images/0186/4128/967c8.png)
![[NOBr]=\frac{0.412mol}{10.3L}](/tpl/images/0186/4128/ea500.png) = 0.040 M
= 0.040 M![[NO]=\frac{0.678mol}{10.3L}](/tpl/images/0186/4128/0105d.png) = 0.0658 M
= 0.0658 M![[Br_2]=\frac{0.224mol}{10.3L}](/tpl/images/0186/4128/3e384.png) = 0.0217 M
= 0.0217 M