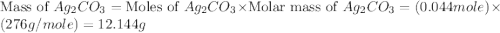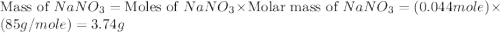Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 4.25 g of sodium carbonate is mixed with one containing 7.50 g of silver nitrate. how many grams of each of the following compounds are present after the reaction is complete?
i) sodium carbonate
ii) silver nitrate
iii) silver carbonate
iv) sodium nitrate

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution...
Questions



Mathematics, 09.10.2019 08:20


Chemistry, 09.10.2019 08:20


Mathematics, 09.10.2019 08:20

Chemistry, 09.10.2019 08:20


Mathematics, 09.10.2019 08:20



Business, 09.10.2019 08:20


Chemistry, 09.10.2019 08:20

Chemistry, 09.10.2019 08:20


Mathematics, 09.10.2019 08:20


English, 09.10.2019 08:20

 ,
,  are, 1.908 g, 0 g, 12.144 g and 3.74 g respectively.
are, 1.908 g, 0 g, 12.144 g and 3.74 g respectively.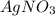 = 7.50 g
= 7.50 g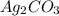 = 276 g/mole
= 276 g/mole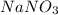 = 85 g/mole
= 85 g/mole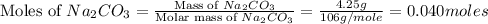
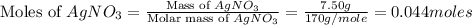
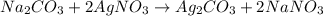
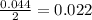 moles of
moles of 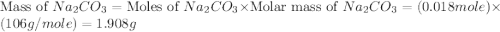
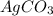 .
.