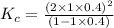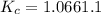Chemistry, 22.08.2019 19:30 tddreviews
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibriumdescribed by the equation n2o4(g)↔ 2no2(g). if at equilibrium then2o4is 40.% dissociated, what is the value of the equilibriumconstant (in units of moles per liter) for the reaction under theseconditions?
a. 0.20
b. 0.84
c. 1.1
d. 1.5
e. 2.0

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

You know the right answer?
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibriumdescri...
Questions


Computers and Technology, 22.03.2021 23:00


Mathematics, 22.03.2021 23:00





Health, 22.03.2021 23:00

Mathematics, 22.03.2021 23:00


Biology, 22.03.2021 23:00


Mathematics, 22.03.2021 23:00


Mathematics, 22.03.2021 23:00

Mathematics, 22.03.2021 23:00


World Languages, 22.03.2021 23:00

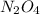 = 1.0 mole
= 1.0 mole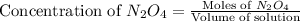
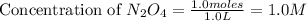
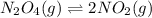
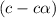
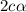
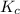 will be,
will be,![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0188/7267/271f5.png)
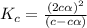
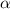 = degree of dissociation = 40 % = 0.4
= degree of dissociation = 40 % = 0.4