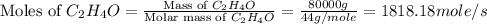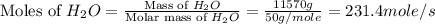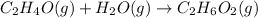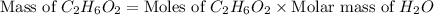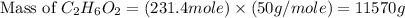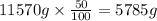Chemical reactions and stoichiometry q2. ethylene glycol (c2hs02) can be produced from the reaction of ethylene oxide (c2h40) and water as shown below. 2h40(g) t 20(g) c2h602(0) if 80 kg/s of c2h40 is mixed with 25.5 lbm/s of water, a) what is the limiting reactant? b) how much ethylene glycol is produced when the reaction is complete? c) how much ethylene glycol is produced if c2h40 is 50% pure

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement accurately describes the rock layers? layer 8 is older than layer 1. layer 3 is younger than layer 6. layer 4 and layer 10 are the same relative age. layer 2 and layer 9 are the same relative age.
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
Chemical reactions and stoichiometry q2. ethylene glycol (c2hs02) can be produced from the reaction...
Questions

Computers and Technology, 12.04.2021 21:00

Mathematics, 12.04.2021 21:00


English, 12.04.2021 21:00

Mathematics, 12.04.2021 21:00




Mathematics, 12.04.2021 21:00



Mathematics, 12.04.2021 21:00

History, 12.04.2021 21:00

Computers and Technology, 12.04.2021 21:00


History, 12.04.2021 21:00


Mathematics, 12.04.2021 21:00


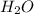
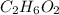 is, 11570 grams
is, 11570 grams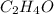 = 80 kg/s = 80000 g/s
= 80 kg/s = 80000 g/s