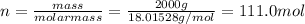Chemistry, 27.08.2019 17:10 Jackiebear4593
Assuming the mass of the ice in the beaker was 2000 g (2 kg), calculate the amount of heat that must have been added to convert the ice to water (assume that the entire 2000 g was ice at the start of melting, and the entire 2000 g was water at the end of the melting). assume that the change took place entirely at 0 °c. the heat of fusion (dhfus) for h2o is 6.12 kj/mol.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
Assuming the mass of the ice in the beaker was 2000 g (2 kg), calculate the amount of heat that must...
Questions

English, 05.05.2020 15:03




Social Studies, 05.05.2020 15:03


Social Studies, 05.05.2020 15:03

Chemistry, 05.05.2020 15:03

History, 05.05.2020 15:03



History, 05.05.2020 15:03



Health, 05.05.2020 15:03

Mathematics, 05.05.2020 15:03

Biology, 05.05.2020 15:03

Mathematics, 05.05.2020 15:03


Social Studies, 05.05.2020 15:03