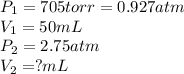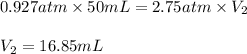Chemistry, 30.08.2019 18:10 brydenskl814
(boyle's law):
1. what is the final volume (in ml) or argon gas is 50.0 ml at 705 torr is compressed to a pressure of 2.75 atm, at constant temperature?

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
(boyle's law):
1. what is the final volume (in ml) or argon gas is 50.0 ml at 705 torr is com...
1. what is the final volume (in ml) or argon gas is 50.0 ml at 705 torr is com...
Questions


Social Studies, 23.07.2019 10:00

Mathematics, 23.07.2019 10:00


Mathematics, 23.07.2019 10:00




Mathematics, 23.07.2019 10:00

Mathematics, 23.07.2019 10:00

Social Studies, 23.07.2019 10:00

Mathematics, 23.07.2019 10:00

Mathematics, 23.07.2019 10:00

Biology, 23.07.2019 10:00

Health, 23.07.2019 10:00


Mathematics, 23.07.2019 10:00


Mathematics, 23.07.2019 10:00

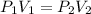
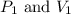 are initial pressure and volume.
are initial pressure and volume.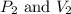 are final pressure and volume.
are final pressure and volume.