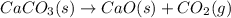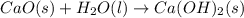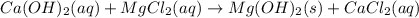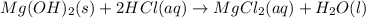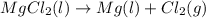Anovel process for obtaining magnesium from sea water involves several reactions. write a balanced chemical equation for each step of the process.
(a) the first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide
(b)the second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water
(c)solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride
(d)thw solid hydroxide is added to a hydrochloric acid solution producing dissolved magnesium chloride and liquid water
(e) finally, the magnesium chloride is melted and electrolyzed metal and diatomic chlorine gas.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

You know the right answer?
Anovel process for obtaining magnesium from sea water involves several reactions. write a balanced c...
Questions

Computers and Technology, 26.11.2019 20:31