
Chemistry, 02.09.2019 20:20 firenation18
From the balanced molecular equation, write the complete onic and net ionic equation for the following:
(a)k2c2o4 + ba(oh)2 - > 2koh + bac2o2
(b)pb(no3)2 + h2so4 -> 2hno3 + pbso4
(c)caco3 + h2so4 = co2 + caso4 + h2o

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
From the balanced molecular equation, write the complete onic and net ionic equation for the followi...
Questions

Mathematics, 12.04.2021 19:20

Mathematics, 12.04.2021 19:20

Physics, 12.04.2021 19:20


History, 12.04.2021 19:20




Mathematics, 12.04.2021 19:20



Mathematics, 12.04.2021 19:20

Business, 12.04.2021 19:20

Mathematics, 12.04.2021 19:20



History, 12.04.2021 19:20

Mathematics, 12.04.2021 19:20


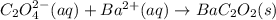
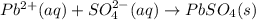
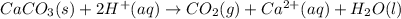
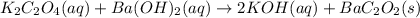
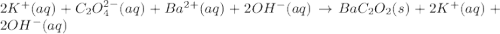
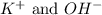 are the spectator ions.
are the spectator ions.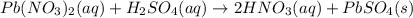
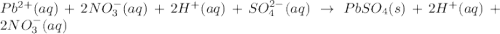
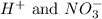 are the spectator ions.
are the spectator ions.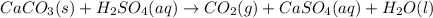
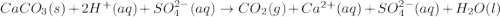
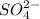 is the spectator ions.
is the spectator ions.


