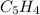Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
The principal component of mothballs is naphthalene, a compound with the molecular mass of about 130...
Questions



Mathematics, 04.10.2021 14:00



Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00





English, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

English, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Computers and Technology, 04.10.2021 14:00

Chemistry, 04.10.2021 14:00

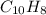 - Molecular formula
- Molecular formula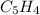 - Empirical formula
- Empirical formula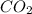 = 10.3 × 10⁻³ g /44.01 g/mol = 0.2340×10⁻³ moles
= 10.3 × 10⁻³ g /44.01 g/mol = 0.2340×10⁻³ moles