Chemistry, 05.09.2019 16:30 lavander9303
Only two stable isotopes of chlorine exist. 35cl has a mass of 34.96885 amu and 37cl has a mass of 36.9659 amu. given that the atomic mass of chlorine is 35.453, calculate the isotopic abundance of 37cl. (enter your answer as a decimal number between zero and one.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Only two stable isotopes of chlorine exist. 35cl has a mass of 34.96885 amu and 37cl has a mass of 3...
Questions

History, 07.06.2021 18:30

Biology, 07.06.2021 18:30


Mathematics, 07.06.2021 18:30

Mathematics, 07.06.2021 18:30

Mathematics, 07.06.2021 18:30


Physics, 07.06.2021 18:30

Mathematics, 07.06.2021 18:30



Mathematics, 07.06.2021 18:30


Mathematics, 07.06.2021 18:30

Health, 07.06.2021 18:30

Mathematics, 07.06.2021 18:30

Chemistry, 07.06.2021 18:30

English, 07.06.2021 18:30


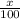
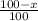
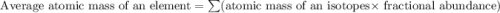
![35.453=\sum[(34.96885 \times \frac{x}{100})+(36.9659 \times \frac{100-x}{100}]]](/tpl/images/0223/4977/3a756.png)