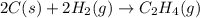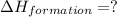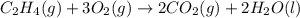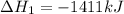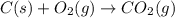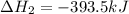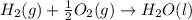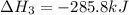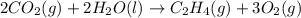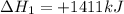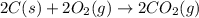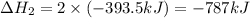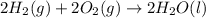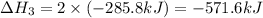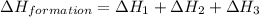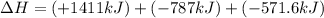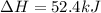Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Find the standard enthalpy of formation of ethylene, c2h4(g), given the following data: c2h4(g) + 3...
Questions

Arts, 24.02.2021 18:50

English, 24.02.2021 18:50

History, 24.02.2021 18:50





Mathematics, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Computers and Technology, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50


Mathematics, 24.02.2021 18:50



Mathematics, 24.02.2021 18:50



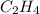 will be,
will be,