
Chemistry, 10.09.2019 18:30 kevinleon695
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas particles have most of their mass concentrated in the nucleus of the atom. b. the moving particles undergo perfectly elastic collisions with the walls of the container. c. the forces of attraction and repulsion between the particles are insignificant. d. the average kinetic energy of the particles is directly proportional to the absolute temperature. e. all of the above are postulates of the kinetic molecular theory.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

You know the right answer?
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas part...
Questions

Mathematics, 14.12.2020 17:20


Mathematics, 14.12.2020 17:20


Mathematics, 14.12.2020 17:20


Physics, 14.12.2020 17:20



Social Studies, 14.12.2020 17:20


Mathematics, 14.12.2020 17:20

English, 14.12.2020 17:20


Mathematics, 14.12.2020 17:20

English, 14.12.2020 17:20



Mathematics, 14.12.2020 17:20

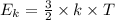 (where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).
(where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).


