

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
When carbon is burned in air, it reacts with oxygen to form carbon dioxide. when 20.4 g of carbon we...
Questions

Mathematics, 26.02.2021 18:00

Biology, 26.02.2021 18:00

Arts, 26.02.2021 18:00


Mathematics, 26.02.2021 18:00

Mathematics, 26.02.2021 18:00



Mathematics, 26.02.2021 18:00

Mathematics, 26.02.2021 18:00




Mathematics, 26.02.2021 18:00



Mathematics, 26.02.2021 18:00



Mathematics, 26.02.2021 18:00

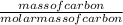
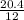 = 1.7moles
= 1.7moles

