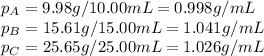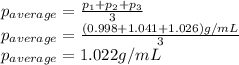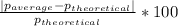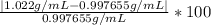Chemistry, 18.09.2019 04:10 christianskyy7074
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0.997655 g/ml. a volume of 10.00 ml of the water had a mass of 9.98 g. a volume of 15.00 ml of the water had a mass of 15.61 g. a volume of 25.00 ml of the water had a mass of 25.65 g. (1) show the calculation of the density of each volume. (2) show the calculation of the average density. (3) show the calculation of the percent error based on the theoretical density of 0.997655 g/ml.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0...
Questions

English, 28.09.2019 04:50


Social Studies, 28.09.2019 04:50









Mathematics, 28.09.2019 04:50


Mathematics, 28.09.2019 04:50



Mathematics, 28.09.2019 04:50

Mathematics, 28.09.2019 04:50

Social Studies, 28.09.2019 04:50