
Valproic acid, used to treat seizures and bipolar disorder, is composed of c, h, and o. a 0.165-g sample is combusted to produce 0.166 g of water and 0.403 g of carbon dioxide. what is the empirical formula for valproic acid? if the molar mass is 144 g> mol, what is the molecular formula?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Valproic acid, used to treat seizures and bipolar disorder, is composed of c, h, and o. a 0.165-g sa...
Questions












Mathematics, 12.04.2021 20:40

History, 12.04.2021 20:40



Mathematics, 12.04.2021 20:40


Spanish, 12.04.2021 20:40


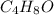
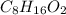
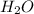 = 0.166 g /18 g/mol = 0.00922 moles
= 0.166 g /18 g/mol = 0.00922 moles
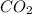 = 0.403 g /44.01 g/mol = 0.009157 moles
= 0.403 g /44.01 g/mol = 0.009157 moles


