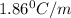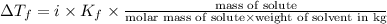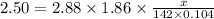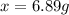Chemistry, 23.09.2019 18:10 pakabigail7116
What mass of na2so4 must be dissolved in 104 grams of water to lower the freezing point by 2.50 °c? the freezing point depression constant, kfp, of water is –1.86 °c/m. assume the van't hoff factor for na2so4 is 2.88.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
What mass of na2so4 must be dissolved in 104 grams of water to lower the freezing point by 2.50 °c?...
Questions



History, 19.08.2019 08:50

History, 19.08.2019 08:50


Chemistry, 19.08.2019 08:50

Mathematics, 19.08.2019 08:50



Mathematics, 19.08.2019 09:00

Mathematics, 19.08.2019 09:00


Mathematics, 19.08.2019 09:00

Mathematics, 19.08.2019 09:00

Mathematics, 19.08.2019 09:00


Mathematics, 19.08.2019 09:00


Mathematics, 19.08.2019 09:00

Social Studies, 19.08.2019 09:00

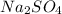 that must be dissolved is 6.89 grams.
that must be dissolved is 6.89 grams.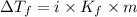
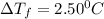 = Depression in freezing point
= Depression in freezing point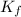 = freezing point constant =
= freezing point constant =