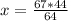The reaction is as follows: ch4 + 202 + co2 + 2h2o if we have 71 kg/hr of ch4 reacting with 67 kg/hr of 02, at what rate co2 will be generated in kg/hr? molecular weight: c-12 kg/kmol h-1 kg/kmol 0 - 16 kg/kmol select one: o a. 46.062 o b. 195.250 o o o c. 1.047 d. 92.125 e. 24.364

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
The reaction is as follows: ch4 + 202 + co2 + 2h2o if we have 71 kg/hr of ch4 reacting with 67 kg/h...
Questions


Mathematics, 18.03.2020 19:31

English, 18.03.2020 19:31

Mathematics, 18.03.2020 19:32


English, 18.03.2020 19:32



English, 18.03.2020 19:32

Social Studies, 18.03.2020 19:32

Mathematics, 18.03.2020 19:32


English, 18.03.2020 19:32


Mathematics, 18.03.2020 19:32

English, 18.03.2020 19:32



Mathematics, 18.03.2020 19:32