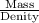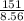Density i
mass
volume of metal
final water volume
step 1
use the metal’s density as a conversion factor to convert from mass (in units of grams) to volume (in units of milliliters).
step 2
the metal displaces its volume in the water so the water level rises by that volume. the final volume is determined by adding the initial volume and the volume of the metal.
you have a 100.0-ml graduated cylinder containing 50.0 ml of water. you carefully place a 151-g piece of brass (density = 8.56 g/ml) into the water. what is the final volume reading in the graduated cylinder?
ml
what is the volume that 151 g of brass occupies?
ml
if the brass displaces this volume of water, what is the final volume in the cylinder?
volume brass = 17.6 ml
ml

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
Density i
mass
volume of metal
final water volume
st...
mass
volume of metal
final water volume
st...
Questions









Chemistry, 29.08.2020 18:01

Computers and Technology, 29.08.2020 18:01



Mathematics, 29.08.2020 18:01



Business, 29.08.2020 18:01