
Chemistry, 02.10.2019 22:00 TropicalFan
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in hz) and wavelength (in nm) of one of these photons.
frequency hz
and
wavelength nm
what is the total energy (in kj) in 1 mole of these photons?
kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

You know the right answer?
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in h...
Questions











Computers and Technology, 11.10.2020 23:01






Biology, 11.10.2020 23:01

Mathematics, 11.10.2020 23:01


Physics, 11.10.2020 23:01

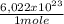 = 230040 J/mole ≡ 230,0 kJ/mole
= 230040 J/mole ≡ 230,0 kJ/mole

