
A5.00-ml sample of blood was treated with trichloroacetic acid to precipitate proteins. after centrifugation, the resulting solution was brought to a ph of 3 and was extracted with two 5-ml portions of methyl isobutyl ketone containing the organic lead complexing agent apcd. the extract was aspirated directly into an air-acetylene flame yielding an absorbance of 0.454 at 283.3 nm. five-milliliter aliquots of standard solutions containing 0.240 and 0.475 ppm pb were treated in the same way and yielded absorbances of 0.412 and 0.642. calculate the concentration pb (ppm) in the sample assuming that beer’s law is followed.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
A5.00-ml sample of blood was treated with trichloroacetic acid to precipitate proteins. after centri...
Questions


Mathematics, 11.03.2020 01:57



Mathematics, 11.03.2020 01:57

History, 11.03.2020 01:57

History, 11.03.2020 01:57

English, 11.03.2020 01:57


Mathematics, 11.03.2020 01:57



Mathematics, 11.03.2020 01:57

English, 11.03.2020 01:57

Health, 11.03.2020 01:57


Mathematics, 11.03.2020 01:57



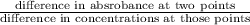
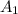 = 0.412 (first point)
= 0.412 (first point)
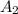 = 0.642 (second point)
= 0.642 (second point)
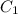 = 0.240 ppm (first point)
= 0.240 ppm (first point)
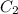 = 0.475 ppm (second point)
= 0.475 ppm (second point)
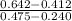
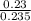
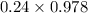 + c
+ c
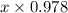 + 0.178
+ 0.178

