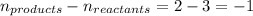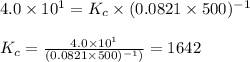The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three gases has partial pressures of 9.10×10−2 atm , 0.174 atm , and 0.24 atm for no, cl2, and nocl, respectively. part apart complete calculate kp for this reaction at 500.0 k. express your answer using two significant figures. kp = 40 previous answers correct part b if the vessel has a volume of 6.00 l , calculate kc at this temperature. express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three...
Questions


Mathematics, 16.12.2020 01:00

History, 16.12.2020 01:00



Mathematics, 16.12.2020 01:00


Mathematics, 16.12.2020 01:00


World Languages, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00


Mathematics, 16.12.2020 01:00

English, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Chemistry, 16.12.2020 01:00



Mathematics, 16.12.2020 01:00

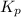 for the given reaction is
for the given reaction is 
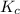 for the given reaction is 1642.
for the given reaction is 1642.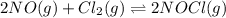
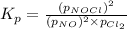
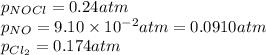
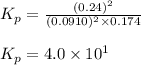
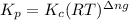
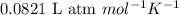
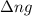 = change in number of moles of gas particles =
= change in number of moles of gas particles =