
Chemistry, 10.10.2019 04:10 emmabarnett817
What is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00 l of 0.0100 m benzoic acid, hc7h5o2? assume that there is no change in volume. the ka for benzoic acid is 6.3 × 10–5. view available hint(s) what is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00 l of 0.0100 m benzoic acid, hc7h5o2? assume that there is no change in volume. the ka for benzoic acid is 6.3 × 10–5. 4.33 3.76 0.439 4.64

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3


Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
What is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00...
Questions


Chemistry, 05.01.2021 05:50

English, 05.01.2021 05:50







History, 05.01.2021 06:00

Health, 05.01.2021 06:00


Mathematics, 05.01.2021 06:00



Social Studies, 05.01.2021 06:00


Mathematics, 05.01.2021 06:00

Mathematics, 05.01.2021 06:00

Mathematics, 05.01.2021 06:00

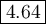
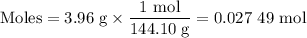
![[\text{A}^{-}] = \dfrac{\text{0.027 49 mol}}{\text{1 L}} = \text{0.027 49 mol/L }](/tpl/images/0305/8117/b8b4c.png)
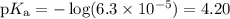
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log\dfrac{[\text{A}^{-}]}{\text{[HA]}}\\\\& = & 4.20 +\log\dfrac{0.02749}{0.0100}\\\\& = & 4.20 + \log2.749 \\& = & 4.20 + 0.4390\\& = & 4.64\\\end{array}\\\text{The pH of the buffer is $\large \boxed{\mathbf{4.64}}$}](/tpl/images/0305/8117/4e2e9.png)


