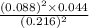Chemistry, 12.10.2019 03:10 shradhwaip2426
At a certain temperature, 0.760 mol so3 is placed in a 2.50 l container. 2so3(g)↽−−⇀2so2(g)+o2(g) at equilibrium, 0.110 mol o2 is present. calculate kc.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
At a certain temperature, 0.760 mol so3 is placed in a 2.50 l container. 2so3(g)↽−−⇀2so2(g)+o2(g) at...
Questions

Geography, 24.09.2019 07:30

Mathematics, 24.09.2019 07:30


Computers and Technology, 24.09.2019 07:30

Mathematics, 24.09.2019 07:30

Spanish, 24.09.2019 07:30


Physics, 24.09.2019 07:30





English, 24.09.2019 07:30


History, 24.09.2019 07:30


History, 24.09.2019 07:30

Mathematics, 24.09.2019 07:30


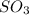 = 0.760 mol
= 0.760 mol
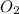 present at equilibrium = 0.110 mol
present at equilibrium = 0.110 mol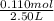
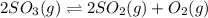
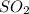 = 2x
= 2x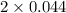
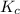 ) as follows.
) as follows.![K_{c} = \frac{[SO_{2}]^{2}[O_{2}]}{[SO_{3}]^{2}}](/tpl/images/0312/0764/e07bc.png)