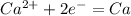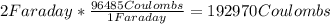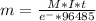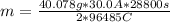Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

You know the right answer?
How many grams of ca metal are produced by the electrolysis of molten cabr2 using a current of 30.0...
Questions

Health, 20.06.2019 18:04


Geography, 20.06.2019 18:04


Computers and Technology, 20.06.2019 18:04






Mathematics, 20.06.2019 18:04









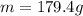 of Ca
of Ca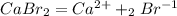
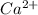 ion will be:
ion will be: