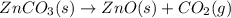The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 71.5 kj of heat per mole of znco3. you may want to reference (pages 177 - 178) section 5.4 while completing this problem. part apart complete write a balanced equation for the reaction. express your answer as a chemical equation. identify all of the phases in your answer. znco3(s)→zno(s)+co2(g) previous answers correct the reactant and products of the decomposition reaction are given in the introduction. znco3(s) is broken down into zno(s) and co2(g). part b what is the enthalpy of the reaction above?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 7...
Questions




Mathematics, 22.02.2021 04:20


Mathematics, 22.02.2021 04:20

Advanced Placement (AP), 22.02.2021 04:20

Physics, 22.02.2021 04:20

Mathematics, 22.02.2021 04:20

History, 22.02.2021 04:20








Mathematics, 22.02.2021 04:20