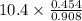Chemistry, 18.10.2019 19:10 Chrissyx4750
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in part a, and the temperature and volume were constant at values of 303 k and 2.00 l, respectively. if the pressure was 10.4 atm prior to the reaction, what would be the expected pressure after the reaction was completed?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

You know the right answer?
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in par...
Questions


Biology, 11.05.2021 18:50



Physics, 11.05.2021 18:50


Social Studies, 11.05.2021 18:50

English, 11.05.2021 18:50

Mathematics, 11.05.2021 18:50

Mathematics, 11.05.2021 18:50



Biology, 11.05.2021 18:50





Biology, 11.05.2021 18:50

Mathematics, 11.05.2021 18:50

History, 11.05.2021 18:50

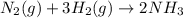
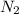 + moles of
+ moles of 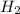 = 0.908 mol
= 0.908 mol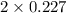 = 0.454 mol
= 0.454 mol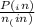 =
= 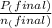
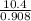 =
= 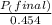
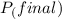 =
=