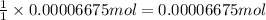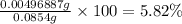Chemistry, 18.10.2019 22:00 hei40563273
In part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample of household bleach is completely reacted with ki(s). the resulting solution is then titrated with 0.150 m nas2o3 solution. 0.890 ml of the solution is required to reach the colorless endpoint. what is the mass percent of naocl (mm = 74.44 g/mole) in the bleach? a. 30.1% b. 96.5% c. 2.23% d. 5.82%

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1


Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 18:10
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? particle spacing can allow a very high density. particle kinetic energy is independent of temperature. particles vibrate quickly in stationary positions. particles exchange energy through elastic collisions.
Answers: 2
You know the right answer?
In part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample o...
Questions






Mathematics, 24.04.2021 08:00

Mathematics, 24.04.2021 08:00

English, 24.04.2021 08:00



Mathematics, 24.04.2021 08:00


History, 24.04.2021 08:00

Chemistry, 24.04.2021 08:00


Biology, 24.04.2021 08:00


Mathematics, 24.04.2021 08:00


History, 24.04.2021 08:00

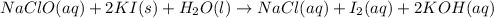 ..[1]
..[1]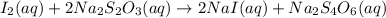 ..[2]
..[2]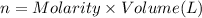
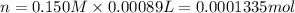
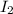 .
.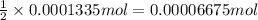 of
of