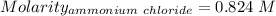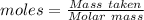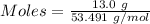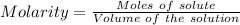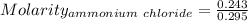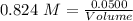Asolution is created by dissolving 13.0 grams of ammonium chloride in enough water to make 295 ml of solution. how many moles of ammonium chloride are present in the resulting solution? =0.243 moleswhen thinking about the amount of solute present in a solution, chemists report the concentration or molarity of the solution. molarity is calculated as moles of solute per liter of solution. what is the molarity of the solution described above? =0.824 mto carry out a particular reaction, you determine that you need 0.0500 moles of ammonium chloride. what volume of the solution described above will you need to complete the reaction without any leftover nh4cl? ml of solution?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
Asolution is created by dissolving 13.0 grams of ammonium chloride in enough water to make 295 ml of...
Questions







Mathematics, 21.01.2020 01:31

Mathematics, 21.01.2020 01:31

Mathematics, 21.01.2020 01:31


Business, 21.01.2020 01:31