Chemistry, 22.10.2019 03:20 bubbaforbes2005
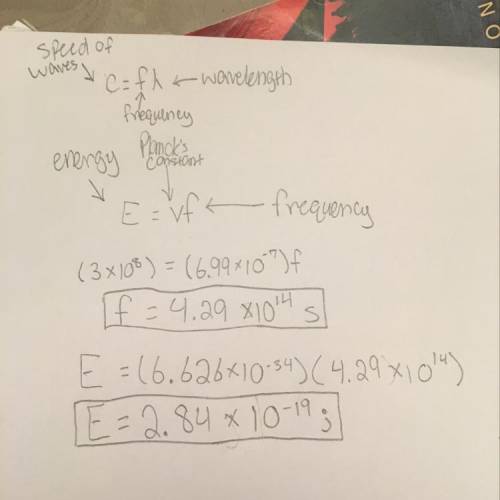
Assume that wave #1 has a wavelength of 699 nm. calculate the frequency and energy of the wave (show your work).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Assume that wave #1 has a wavelength of 699 nm. calculate the frequency and energy of the wave (show...
Questions

Chemistry, 29.09.2019 01:10

History, 29.09.2019 01:10


Medicine, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10


Mathematics, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10



Mathematics, 29.09.2019 01:10


Mathematics, 29.09.2019 01:10

Engineering, 29.09.2019 01:10


Engineering, 29.09.2019 01:10

Engineering, 29.09.2019 01:10