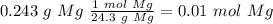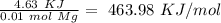When 0.243 g of mg metal is combined with enough hcl to make 100 ml of solution in a constant-pressure calorimeter, the following reaction occurs: mg1s2+2 hcl1aq2¡mgcl21aq2+h21g2if the temperature of the solution increases from 23.0 to 34.1 °c as a result of this reaction, calculate ∆h in kj> mol mg. assume that the solution has a specific heat of 4.18 j> g@°c and

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
When 0.243 g of mg metal is combined with enough hcl to make 100 ml of solution in a constant-pressu...
Questions


Mathematics, 09.07.2019 07:10


Mathematics, 09.07.2019 07:10



Social Studies, 09.07.2019 07:10

Mathematics, 09.07.2019 07:10


Mathematics, 09.07.2019 07:10


Mathematics, 09.07.2019 07:10

Mathematics, 09.07.2019 07:10


Mathematics, 09.07.2019 07:10


Mathematics, 09.07.2019 07:10


SAT, 09.07.2019 07:10

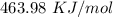
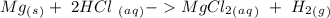
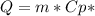 ΔT
ΔT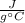
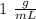
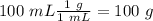
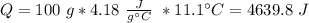
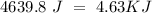
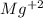 , so:
, so: