
Chemistry, 23.10.2019 18:00 krishawnnn
For the generic equilibrium ha(aq) ⇌ h+(aq) + a−(aq), which of these statements is true? for the generic equilibrium , which of these statements is true? if you add the soluble salt ka to a solution of ha that is at equilibrium, the concentration of ha would decrease. if you add the soluble salt ka to a solution of ha that is at equilibrium, the ph would increase. the equilibrium constant for this reaction changes as the ph changes. if you add the soluble salt ka to a solution of ha that is at equilibrium, the concentration of a− would decrease.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
For the generic equilibrium ha(aq) ⇌ h+(aq) + a−(aq), which of these statements is true? for the ge...
Questions



Physics, 15.07.2019 14:30



Chemistry, 15.07.2019 14:30

Computers and Technology, 15.07.2019 14:30



Business, 15.07.2019 14:30

Business, 15.07.2019 14:30

Business, 15.07.2019 14:30




Social Studies, 15.07.2019 14:30

Business, 15.07.2019 14:30

Social Studies, 15.07.2019 14:30


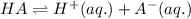
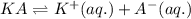
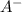 ion is getting increased on the product side, so the equilibrium will shift in the direction to minimize this effect, which is in the direction of HA.
ion is getting increased on the product side, so the equilibrium will shift in the direction to minimize this effect, which is in the direction of HA.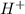 ions are getting decreases. This will increase the pH of the solution.
ions are getting decreases. This will increase the pH of the solution.



