Chemistry, 25.10.2019 19:43 niceguy1997
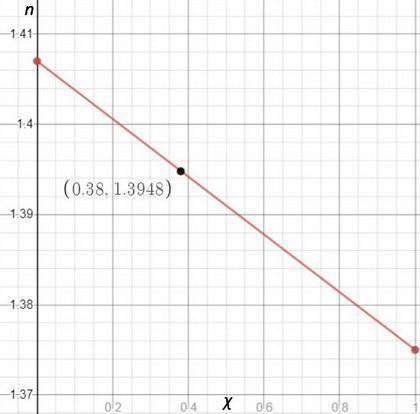
You are performing a simple distillation of roughly 50: 50 liquid solution containing two components, hexane and nonane you place 15 ml of the mixture in a round bottom flask, and then collect the distillate sequentially as four three ml samples labeled s1, s2, s3, and s4. pure hexane has a refractive index of 1.375 and pure nonane has a refractive index of 1.407. you measure a refractive index of 1.3948 for one of the four samples. assuming the refractive index varies linearly with mole fraction, estimate the mole fraction of hexane in this sample.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
You are performing a simple distillation of roughly 50: 50 liquid solution containing two components...
Questions



Chemistry, 27.06.2019 17:30

Mathematics, 27.06.2019 17:30


Mathematics, 27.06.2019 17:30


Mathematics, 27.06.2019 17:30

Mathematics, 27.06.2019 17:30






Biology, 27.06.2019 17:30