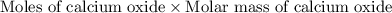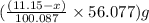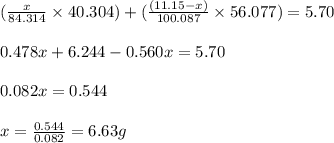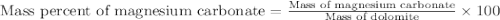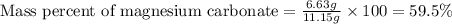Chemistry, 26.10.2019 00:43 mckadams02
Dolomite is a mixed carbonate of calcium and magnesium that decomposes to co2 and the metal oxides mgo and cao upon heating. when 11.15 g of dolomite is heated, 5.70 g of mgo and cao are produced. what is percent by mass of mgco3 in the original sample of dolomite?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Dolomite is a mixed carbonate of calcium and magnesium that decomposes to co2 and the metal oxides m...
Questions






Mathematics, 13.07.2019 13:00

Mathematics, 13.07.2019 13:00



Mathematics, 13.07.2019 13:00

Mathematics, 13.07.2019 13:00

Mathematics, 13.07.2019 13:00

Mathematics, 13.07.2019 13:00


History, 13.07.2019 13:00



Business, 13.07.2019 13:00


English, 13.07.2019 13:00

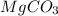 in dolomite is 59.5 %
in dolomite is 59.5 %
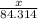 moles
moles 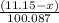 moles
moles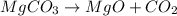
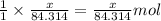 of magnesium oxide
of magnesium oxide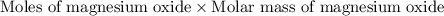
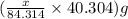

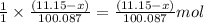 of calcium oxide
of calcium oxide