
Chemistry, 26.10.2019 04:43 Halessoftball
What is the molar concentration of fe2+ ion in an aqueous solution if 37.50 ml of 0.118 m kbro3 is required for complete reaction with 11.00 ml of the fe2+ solution? the net ionic equation is: 6fe2+(aq)+bro3−(aq)+6h+(aq)→6fe3+(a q)+br−(aq)+3h2o(l).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
What is the molar concentration of fe2+ ion in an aqueous solution if 37.50 ml of 0.118 m kbro3 is r...
Questions




















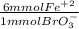 = 26.55 mmol Fe²⁺
= 26.55 mmol Fe²⁺

