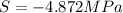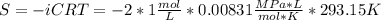Chemistry, 29.10.2019 21:31 tynyiaawrightt
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential of the soil at 20°c using the solute potential equation: ѱs = –icrt where i is the ionization constant (2 for nacl), c is the molar concentration (in mol/l), r is the pressure constant [r = 0.00831 l • mpa/(mol • k)], and t is the temperature in kelvin (273 + °c). how much will the solute potential of the soil be lowered at 20°c?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential...
Questions

English, 29.01.2020 05:40

Chemistry, 29.01.2020 05:40


Mathematics, 29.01.2020 05:40

Mathematics, 29.01.2020 05:40

Mathematics, 29.01.2020 05:40



Mathematics, 29.01.2020 05:40

Chemistry, 29.01.2020 05:40


Mathematics, 29.01.2020 05:40


Mathematics, 29.01.2020 05:40


Mathematics, 29.01.2020 05:40