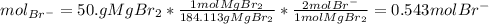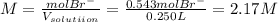Achemist prepares a solution of magnesium bromide by measuring out of into a volumetric flask and filling to the mark with distilled water. calculate the molarity of anions in the chemist's solution. be sure your answer is rounded to the correct number of significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
Achemist prepares a solution of magnesium bromide by measuring out of into a volumetric flask and fi...
Questions

Mathematics, 31.03.2020 16:48




Spanish, 31.03.2020 16:48





Biology, 31.03.2020 16:49

History, 31.03.2020 16:50


Biology, 31.03.2020 16:51

History, 31.03.2020 16:51


English, 31.03.2020 16:54



Social Studies, 31.03.2020 16:58

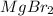 and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into:
and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into: