
Chemistry, 02.11.2019 06:31 xxbloomxx184p8jqjd
2.122 g of a solid mixture containing only potassium carbonate (fw = 138.2058 g/mol) and potassium bicarbonate (fw = 100.1154 g/mol) is dissolved in distilled water. 34.16 ml of a 0.762 m hcl standard solution is required to titrate the mixture to a bromocresol green end point. calculate the weight percent of potassium carbonate and potassium bicarbonate in the mixture.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1


Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
2.122 g of a solid mixture containing only potassium carbonate (fw = 138.2058 g/mol) and potassium b...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








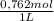 = 0,02603 mol of HCl
= 0,02603 mol of HCl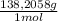 + Y×
+ Y×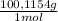 (1)
(1)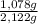 ×100 = 50,8 wt%
×100 = 50,8 wt%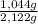 ×100 = 49,2 wt%
×100 = 49,2 wt%

