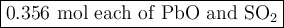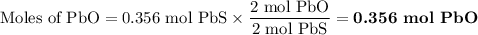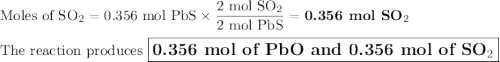2. for the following reaction, calculate how many moles of each product are formed when 0.356
...


Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Questions



Mathematics, 15.01.2021 06:30

Mathematics, 15.01.2021 06:30



Biology, 15.01.2021 06:30

Mathematics, 15.01.2021 06:30



Mathematics, 15.01.2021 06:30

History, 15.01.2021 06:30

Physics, 15.01.2021 06:30







Mathematics, 15.01.2021 06:30