
Asolution of 0.207 m aspartic acid, the charge neutral form of the amino acid, is titrated with 0.0690 m naoh . the pka values for aspartic acid are 1.990 , 3.900 , and 10.002 , corresponding to the α-carboxylic acid group, the β-carboxylic acid group, and the amino group, respectively. calculate the ph at the first equivalence point of this titration.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

You know the right answer?
Asolution of 0.207 m aspartic acid, the charge neutral form of the amino acid, is titrated with 0.06...
Questions

Mathematics, 04.04.2021 15:30


Chemistry, 04.04.2021 15:30

English, 04.04.2021 15:30

Social Studies, 04.04.2021 15:30

Mathematics, 04.04.2021 15:40



Mathematics, 04.04.2021 15:40

History, 04.04.2021 15:40



Social Studies, 04.04.2021 15:40

Geography, 04.04.2021 15:40


World Languages, 04.04.2021 15:40

English, 04.04.2021 15:40

Social Studies, 04.04.2021 15:40

Mathematics, 04.04.2021 15:40

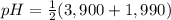 = 2,945
= 2,945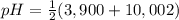 = 6,951
= 6,951


