
Chemistry, 08.11.2019 06:31 kaziyahf2006
Amixture of methane gas, ch4(g) , and pentane gas, c5h12(g) , has a pressure of 0.5799 atm when placed in a sealed container. the complete combustion of the mixture to carbon dioxide gas, co2(g) , and water vapor, h2o(g) , was achieved by adding exactly enough oxygen gas, o2(g) , to the container. the pressure of the product mixture in the sealed container is 2.378 atm . calculate the mole fraction of methane in the initial mixture, assuming the temperature and volume remain constant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

You know the right answer?
Amixture of methane gas, ch4(g) , and pentane gas, c5h12(g) , has a pressure of 0.5799 atm when plac...
Questions

History, 03.02.2020 00:50


Chemistry, 03.02.2020 00:50

Mathematics, 03.02.2020 00:50


Mathematics, 03.02.2020 00:51

Health, 03.02.2020 00:51



History, 03.02.2020 00:51


Mathematics, 03.02.2020 00:51



Spanish, 03.02.2020 00:51

Mathematics, 03.02.2020 00:51


Spanish, 03.02.2020 00:51

Mathematics, 03.02.2020 00:51

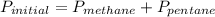
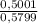 = 0,8624
= 0,8624

